Material Science Group's Newsletter-2018

Editorial Board
Dr. Abdul Majid (Group Leader)
Ms. Amber Batool (Editor)
Letter from the Chair
Head of Material Science Group
The last decade has been exciting part of life due to active involvement in research as it given me a sense of contribution either to basics of Physics or to different applications. The research, either experimental or theoretical, is not only an indicator of hope for humanity but also strengthening the human supremacy on other creations of God. The experimentalists are practically producing realities whereas theoreticians are uncovering the scientific phenomenon through modeling, analysis and first principles methods. Having worked on top class experimental facilities as well as enjoying ab-initio quantum mechanical computations on world’s best equipment, then a luxury that cannot be afforded in third world countries.
I am convinced by resourcefulness of theoretical methods. In the current regime of touching the Moor’s law limits, the availability of sophisticated equipment in a laboratory to meet the technological challenges is not less a luxury that cannot be afforded in third world countries.
Contrary to this, thanks to recent progress in computer hardware and improved programming tools, the members of my research group have access to reasonable computational facilities in Computational Physics Lab of the department. They are working hard to research on cutting edges on physics, material science and allied disciplines in the pursuit of improving rechargeable ion batteries, dye sensitized solar cells and other energy applications. Besides, these the work on semiconducting, magnetic, optical and electronic materials is also in progress. It is hoped that the research and academic activities in this group will not only contribute towards national progress but also to international community. The recent successes speak louder than the words.
The department of Physics and Vice Chancellor, University of Gujrat is acknowledged for providing all possible facilities. May Allah bless all of us.
Current Researchers
Mr. Irslan Ullah Ashraf
Mr. Irslan Ullah is currently working as Lecturer Physics in department of Physics, University of Gujrat, Pakistan. He is also working as Research Associate in HEC funded Research Project “Computational Study of TM:SiC Alloys to Design Efficient Material for Counter Electrodes to be Used in Dye Sensitized Solar Cells” with PI. He did M. Phil in Physics from same department while working in the field of energy storage devices and materials. His attempted to improve the performance of Li ion batteries using computational techniques mainly DFT and Molecular Dynamics. Prior to this, he earned B.S. in Physics from same department.

Mr. Waqas-ul-Hassan
I am Muhammad Waqas Ul Hassan, I am doing Master of Philosophy in Physics in University of Gujrat, (Gujrat) Pakistan. I have recently submitted my thesis on the topic “First Principles Study LiCePO4 for Upgradation of Cathode Material in Lithium Ion Batteries”. Research duration experience under kind supervision and guidance of Dr. Abdul Majid. In fact, it is best approach to approximate materials as well as their electronic and magnetic properties. We take complex systems and deal with them simply with the help of Schrodinger’s Equation and check whether the material can be utilized in further experiments. It seems like “A Computational researcher can solve any problem of this world.

Mr. Muhammad Saim Bukhari
Mr. Saim Bukhari has recently done with MPhil Physics, from University of Gujrat Pakistan. As an MPhil scholar he worked under the supervison of Dr Abdul Majid Sandhu and worked on TM doped SnO to find its applications in Thin film transistors, LCDs, LED,s etc. His contributions for his group always been honored by respected chair. Not only chair of this group all other members are supposrting me in this regard. I must want to say that DFT lab is onre of the big source of knowledge for UOG research students.

Ms. Saddiqa Habib
My Research period was astonishing and informative under the devoted guidance of Dr. Abdul Majid whose guidance attracted and motivated me to work keenly with gain of enormous concepts which in turn developed interest to move on further in research field. I had confusing part which was that why there is a gap between available and substantiated computational methodologies and their usage in computational physics moreover, how can Computational Physics Community at large cope with recent accelerated progress of every scientific fields acting as Mathematical Basis? and so on
Unlike No, doubt DFT always has been a vital part in Research World.

Ms. Alia Jabeen
Miss Alia Jabeen is currently doing her MPhil from University of Gujrat. Prior to this, she did her M.Sc in Physics from same department while working on structural, vibrational and optical properties of the hybrid clusters that is cis- and trans- and worked under the supervision of Dr. Abdul Majid Sandhu. Currently, she is focusing on the basic mechanism behind water splitting and effect of magnetic field on water splitting. She wants to verify the mechanism described in the literature and model a system to study and investigate the water splitting by using ADF or VASP code. Her research interest includes the theoretical study of the material sciences and also keen to learn the basic mechanism behind the DFT and DFT Calculations.

Ms. Amber Batool
Miss Amber Batool is theorist working on Topological insulators. She did M.Sc in Physics from university of Gujrat while working to investigate the clusters. Her attempted to improve the understanding of structural, vibrational and optical behavior of TiSiO4 hybrid cluster using DFT in ADF code under the supervision of Dr. Abdul Majid Sandhu. Her research interests include computational study of material science with focus on semiconductors, energy materials and devices. She got 8th position in Photo contents “Science in Nature” at international level held by Gull’s Association. Currently she is perusing M.phil , she is focusing on 2D topological insulators that promise an avenue to realize fascinating application such as dissipation less transport, spintronic, optoelectronics, thermos-electronics and fault-tolerant quantum computing. She wants to develop more promising topological insulating material and investigate electronic, spin-momentum locked topological edge states and spin orbit coupling in 2D predicted topological insulators. Observation of Dirac cone in the band structure means a lot in spintronics applications.

Research Codes
- ADF (ADF, Band, DFT-B, REXFF) www.scm.com
- COMSOL COMSOL
- VASP VASP
Project
Computational study of TM-SiC alloys to design efficient material for counter electrodes to be used in dye sensitized solar cells (DSSC) 2017-2019
National Research Project for Universities (NRPU)` 6509/Punjab/NRPU/R&D/HEC/2016
The above mentioned project falls in the category of Energy devices, the Dr. Abdul Majid was Principle investigator (PI) of said project.
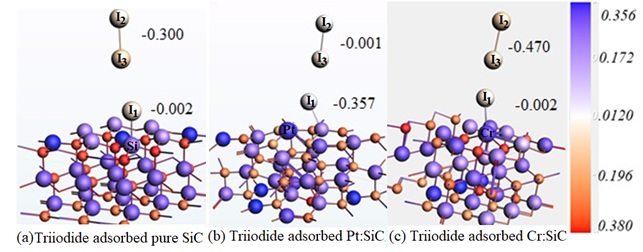
Keeping the future demands of dye sensitized solar cells (DSSC) into account, they choose to work on counter electrode (CE, )The CE is composed of Pt materials which are very expensive and have limited natural abundance. They project outcome was expected to provide practical framework to the experimentalists and industry to hit the nail and fabricate cost effective, abundant and Pt-free CE materials for future DSSCs. For this out group plan to model the catalytic activity in the electrolyte for pure SiC material firstly and then after doping with list of transition metals later on. The catalytic activity of CE was determined by the tri iodide reduction reaction on the surface of respective CE electrode material and the calculated adsorption energy followed by charge transfer analysis determined the catalytic activity.
Group Activities
Workshops Attended by several Group Members
5th National Workshop on Modeling and Simulation of Materials Density Functional Theory (DFT)
A three-day workshop on Modeling and Simulation of Materials using Density Functional Theory (DFT) was organized by Theoretical Physics Division (TPD) and Physics Division (PD) from October 02-04, 2018 in PINSTECH. In speakers Dr. Shafqat Hussain Shah, Dr. Arshad Farhan, Dr. Haqan Shetti, Dr. Akhtar Hussain, Dr.Saqib Javed and Dr. Gul Rehman were confirmed. The workshop focused on the importance of simulation in material science and to disseminate the knowledge regarding various computational material modeling approaches especially Density Functional Theory (DFT). Modeling and simulation of materials is an emerging area of research with a variety of applications from computational, VASP, 2D materials, catalysis, material design to optimize a desired material property for a particular application. It was fifth workshop of its kind, where focus was on introduction of various computational methodologies for material science and engineering rather than on the presentation of published results. Lab Practice sessions were also conducted in the afternoon. This workshop was an effort to highlight the expertise of scientists working in this important research area and to develop collaboration with universities and other strategic organizations. Thirty-six participants including scientists from various strategic organizations, faculty members and students from different universities including university of Gujrat participated in the workshop. In the concluding ceremony, Chief Guest; Dr. Qamar-ul-Haque appreciated the organizers for arranging this event.

Group Meetings and Progress Reports
.Monthly and weekly meetings held on specific day, meanwhile monthly and weekly reports send on every Friday night. Meetings chaired by the group head Dr. Abdul Majid, everyone participate deliberately in these meeting with motivate ideas. Moreover, weekly presentations presented by every group member, relating to basics of DFT and their research topics. Workshops and seminars held every year at international level in which researchers are invited from all over the Pakistan. senior researchers are invited as speakers on emerging fields of research in DFT and experimental field too.
Featured Research Work from Researchers
Olivine Phosphate Materials
By Mr. Waqas-ul-Hassan
Olivine phosphates are renowned family of materials used in cathode materials in Lithium ion batteries. Their operating voltage makes them valuable for being used in rechargeable batteries, which is 2.5-4.0 V. This family of materials made their distinction among other cathode materials due to some basic qualities such as cost, time, simplicity as well as their efficiency. These are less expensive having simple structure such as can be fabricated easily. Whenever we talk about efficiency we compare efficiency with other materials. Different classes of cathode materials have been reported in which Chalcogenides, Silicates, Tavorites layered and spinal oxides are remarkable. Researchers reported that halides and sulfides are less inappropriate as they have low electron conductivity and (open circuit voltage) Voc as well. In this way researches on metal oxides gave results in accordance to establish chargeable batteries. On the other hand layered and spinel frame work such as and respectively can give open circuit voltage about 4.0 but this voltage can decompose electrolyte.
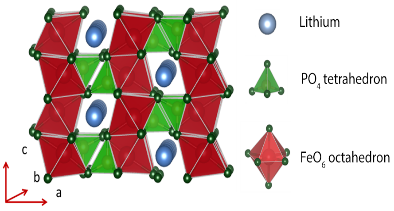
The spinel frame work resists lithium mobility and hence less power density as well as temperature above 180 cathode material decompose permanently from decomposes leaving and . In short olivine phosphates class is best among cathode materials. Lithium intercalation and deintercalation showed that olivine phosphates are efficient as well as more stable in all aspects among these all. Lithium in olivine phosphates have low energy barrier 0.20 eV and hence greater diffusivity in 1 dimension [010]. Electronic conductivity is although low which ultimately reduce the specific capacity of cathode material but this situation is controlled by carbon coating, or adding carbon in olivine structure. LiMPO4 is known as olivine structure having orthorhombic structure in which M is transition metal (Fe, Co, Ni, Mn) connected to corresponding six oxygen atoms making octahedral geometry. Oxygen atoms are strongly bonded with P and Fe atoms making PO4 tetrahedron and FeO6 octahedral geometry. LiMPO4 is stable structure at high temperature which leads good cyclability, long life and safety. As structure is more stable so there is low power density and electronic conductivity which is about 10-13 to 10-16 cm2 s-1 and about 10-9 cm s-1 respectively. Researchers are trying to improve power density and electronic conductivity in these structures. My research topic is also to improve power density while keeping the structure stable. We substitute Fe atoms by Ce atoms at Fe sites. Results are in good agreement. We examine the Electron Localization Function (ELF) and magnetic moments of each atom to check the stability of material.
Two dimensional Dilute Magnetic Semiconductors
By Mr. Muhammad Saim Rafique Bukhari
I have investigated the structural, electronic and magnetic properties of transition metals (TM) Mn, Fe and Cr doped monolayer SnO were investigated based on first principles calculations with aim to design new two-dimensional dilute magnetic semiconductors. The electronic states due to 3d electrons of dopant atoms were found in band gap which played valuable role in spin polarization and band structure modification of the host material. SnO has indirect band gap and its value for Pure SnO is 2.799eV. The calculated values of band gap are 2.000eV, 2.638eV and 2.385 in case of Mn, Fe and Cr doped SnO monolayers respectively and these were direct in nature. The Fermi level was observed near conduction band for the doped materials which rendered TM doped SnO as n-type ferromagnetic semiconductors. The results show that TM dopants are the main source of magnetism in the host material and the localized TM-3d electrons originate as the source of magnetism. The calculated values of magnetic moment per dopant are 4.753μB, 3.581μB and 3.993μB in case of Mn, Fe and Cr doped SnO monolayers respectively. The presence of these inter bands in the band gap due to TM impurity atoms in monolayer SnO suggest their excellent applications in 2D opto-electronics and spintronic device
Physics of Relativistic Effects in theoretical studies
By Ms. Alia Jabeen
Relativistic effects are the disagreement in the value during theoretical studies, whether we consider the relativity or not and this relativity is based on the Einstein’s special theory of relativity. According to which, the mass of a moving object varied from an object at rest, if the object is moving with the velocity relative to that of velocity/speed of light. Mathematically,
m=m0(1-v^2/c^2)
If the fraction is minute then the relativistic effects could be neglected but if this term is larger i.e. speed of an object approaching to speed of light and is equal to the unity then relativistic effects has to be considered. If we consider an electron revolving within the orbit, then according to bohr’s assumption, velocity of the electrons is
V=(2pi e^2/nh)Z
Where V and Z represents the velocity and atomic number respectively. From here, we can say that velocity is directly proportional to the atomic number. Velocity increases as the atomic no increases. It means, that relativistic effects become dominant as the atomic no increases. Hence, for elements having lower atomic number, the relativistic effects could be neglected In fact, the effect of relativity or in simple words relativistic effects increases as . It means it is dominant in the lower part of the periodic table. In various codes based on Quantum mechanics such as ADF, VASP and wien2K, we have the option of “Relativistic effect”. In ADF-Molecule Suit, we have 3 option that is None, Scalar and ZORA. As for smaller atoms the relativistic effects could be neglected, so we mostly choose “None”. We consider the “ZORA (Zero Order Relativistic Approximation)” for the elements having high atomic number and mostly utilize for the Actinides (Ac). But keep in mind, the results are applicable if we consider the special type of the basis sets (in case of ZORA). The results with ZORA and common basis sets are not reliable. For elements having atomic number upto 54-58, we usually consider scaler relativistic effects. VASP usually exhibits the fully relativistic for core electrons and scaler for the valence electrons. In case of GGA+U, the spin orbit along with self-consistent investigation has to be implemented for extensive study and research. The relativistic effects have a great influence on the physical as well as the chemical properties and the peculiar behaviour of some elements are due to the relativistic effects. The liquid form of Hg at room temperature and color of Au could be explained on the basis of relativistic effects.
How cluster science contribute in material Science?
By Ms. Amber Batool
Clusters perform as a bridge between the gas phase and liquid bulk materials. Classification of clusters into elemental or compound clusters and metallic or non-metallic characteristics comprises by the composition and concentration of substitutions. There are three main categories of existing clusters, discrete clusters, magic cluster, and Nano phase materials. Investigation of small clusters is important in the material sciences because it introduces new phases of solids and characteristics interfaces having tunable features. Clusters predict the growth mechanism of the thin surfaces, nanomaterial’s as well as bulk. Characteristics of the clusters based on the material include geometry as well as periodic crystallography. Clusters are helpful in the field of solid state physics; the cluster structures easily detect defects present in the crystals. Within the finite cluster structure, impurity atom interacts with the nearly present cluster atom that seems to be a good model for the periodic band calculation that performed without boundary conditions. Moreover, the optoelectronic properties of cluster allow top use in computers and disk storage devices of a new generation. Shell structure of clusters comprises magnetic properties and energies that resembled by the atomic nuclei, explore more in the nuclear research. Clusters have been considered as the agitated materials since its versatile properties provide a bridge between different fields of physics. Properties of clusters are entirely different from the bulk material such as metals behaves as semiconducting materials, brittleness of materials converts into malleable. Clusters could be described in two categories that is metallic clusters as well as non-metallic clusters based on the type of atoms that are combined to form the clusters. The investigation of the molecular clusters provides a rapid theoretical as well as computational plan in order to approximate the properties of the materials because clusters contains the specific characteristics and act as building blocks of the materials falling in the range of nano, micro as well as in the bulk materials. It has specific applications in catalysis as it has prime applications in the materials adsorbed on the surface. The building blocks of matter commonly found in nature are either atoms or molecules. Depending on the chemistry of the atoms, the ambient pressure, and temperature, the atoms arrange themselves in well-defined arrays to form different crystal lattices. The properties of these materials often depend strongly on how the atoms are arranged. Molecular crystals, on the other hand, exhibit unique properties because molecules, and not atoms, form the building blocks. Like molecular crystals, it is expected that size-selected cluster-assembled materials may possess unique properties hitherto unknown. The central question is how to design and synthesize stable clusters. The criteria depend on the nature of bonding that exists within a cluster. In clusters of simple metals, as outlined previously, the stability is governed by the electronic shell structure. In covalently bonded clusters such as those of C and Si, the atomic arrangements are important for stability. It is also possible to form stable compound clusters by using suitable alloying techniques. In the following, we discuss these cases through illustrative examples. Systematic variation in properties arises due to unusual cluster structures and often effects of boundaries and quantum confinement. In the clusters the magnitude of band gap varies with the composition and size of the cluster atoms. Recently, heterometallic clusters become the demanding materials in the electrocatalysis, photocatalysis, optoelectronic, microelectronic devices. Clusters are attractive materials due to their fascinating properties and offer research activities of interdisciplinary nature. The cluster science provides liberty to prepare new materials and tune the properties for different applications. The clusters behave entirely different from those of bulk materials; metals act like semiconductors and brittle materials transforms into malleable ones. The electronic and optical properties, especially mechanism of charge transfer and electronic transitions are strongly dependent upon these features. The flexibility of tailoring these parameters gives access to infinite possibilities. The metal oxides clusters are extensively used for energy storage applications, batteries, water splitting, catalysis etc.
Future Research Directions
- Illustration of dual nature of Light
- Lithium ion Batteries
- Solar cells
- DSSC
- Organic and inorganic dyes
- Topological Insulator
- 2D layer
- Medical Physics
- Computational study of DNA, Enzymes, Proteins
List of Publications
- A review on First principles based studies for improvement of cathode material of lithium ion batteries , Arslan Ullah, Abdul Majid, Naema Rani, Journal of Energy Chemistry 27, 219 (2018)
- A Review on Novel Eco-Friendly Green Approach to Synthesis TiO2 Nanoparticles Using Different Extracts , Ghulam Nabi, Qurat‑ul‑Aain, N. R. Khalid, M. Bilal Tahir, Muhammad Rafique, Muhammad Rizwan, Sajad Hussain · Tahir Iqbal, Abdul Majid Journal of Inorganic and Organometallic Polymers and Materials, 28, 1552–1564 (2018)
- Characterization of ion irradiated silicon surfaces ablated by laser-induced breakdown spectroscopy , T Iqbal, M Abrar, M B Tahir, M Seemab, A Majid, and S Rafique Chin. Phys. B 27, 087400 (2018)
- Mobility and perpendicular magnetic anisotropy in electrodeposited Co32Fe67B1 thin films using boric acid as boron source , Naeem Ahmad, Fahad Hassan, Suleman Khan, Abdul Majid, Affan Safeer, Ahmad Saeed, Imran Murtaza, A.S. Syed, X.F. Han, Journal of Magnetism and Magnetic Materials 458 (2018), 156-163
- Electrochemical properties of PANI/MoS2 nanosheet composite as an electrode materials , M. Maqsood, Seemab Afzal, Abdul Shakoor, Niaz Ahmad Niaz, Abdul Majid, Najamal Hassan, Hira Kanwal, Journal of Materials Science: Materials in Electronics, 29, 16080–16087 (2018)
- A computational Study of Ferromagnetic Exchange Interactions and Charge Transfer in Codoped Gallium Nitride , Abdul Majid, Naeem Ahmad, Tahir Iqbal Awan, Mehreen Javed Journal of Superconductivity and Novel Magnetism, 31, 745 (2018)
- Effects of Mn Ion Implantation on XPS Spectroscopy of GaN Thin Films , Abdul Majid, Naeem Ahmad, Muhammad Rizwan, Salah Ud-Din Khan, Fekri Abdulraqe Ahmed Ali, Jianjun Journal of Electronic Materials, 47, 1555 (2018)
- A perspective on non-stoichiometry in silicon carbide , Abdul Majid, Ceramics International, 44, 1277 (2018)
- Morphology Tailored Synthesis Of C-WO3 Nanostructures And Its Photocatalytic Application , M. B. Tahir, Ghulam Nabi, A. Hassan, T. Iqbal, H. Kiran, A. Majid, J Inorg Organomet Polym 28, , 738–745 (2018)
